As the Gumboro virus is a very persistent virus and can easily survive in the environment, complete Gumboro control is only possible with an application of an efficacious and well applied Gumboro vaccine in broilers and layers.
This should be implemented alongside a strong focus on cleaning and disinfection, and a solid breeder vaccination program to provide Maternally Derived Antibodies (MDA) in order to prevent early infection of the field Gumboro virus.
Active immunity (or vaccine immunity) induced by the administration of a vaccine will develop according to the vaccine(s) employed, the quality of the application, and the immune status of the chickens at the time of the vaccination.
In order to control Infectious Bursal Disease, several vaccine options are widely available and used:
Inactivated (or killed) Gumboro vaccines
Inactivated (or killed) Gumboro vaccines contain a high amount of inactivated whole or subunit IBD virus presented in a mineral oil emulsion. These vaccines are used to boost MDA in breeders. This way the breeders will transfer a higher level of maternal delivered antibodies to the progeny.
Conventional live attenuated Gumboro vaccines are live attenuated Gumboro viruses that replicate in the bursa of Fabricius, resulting in immunity generated by the replication of the whole virus. There are different Gumboro disease virus strains used in the vaccines and there are different levels of attenuation: Vaccine types are categorised into four groups:
- 'Mild' which are highly attenuated
- 'Intermediate' which are very attenuated
- 'Intermediate Plus' which are moderately attenuated
- 'Hot' which are poorly attenuated
For this type of vaccine, the MDA levels should be monitored. If given too early, in the presence of an excessively high level of MDA, the virus in the vaccine is neutralised or its replication is delayed. If it is given too late, a window of opportunity (also called the protection gap) is offered to the farm virus to infect the flock. This optimal timing depends on the level of MDA and the invasiveness of the vaccine, that is, its capacity to overcome a given titer of MDA.
Vector IBD vaccines
Vector IBD vaccines are constructed from a genetically engineered virus (the vector) whose genome contains a gene from a specific IBDV (the donor) encoding for the VP2 capsid protein. As of today, the Herpes Virus of Turkey (HVT) is mainly used as a vector. Although these vaccines provide proper protection against clinical signs of IBDV, they do not fully colonise the bursa, leading to field IBD viruses being able to enter and replicate in the bursa.
Using a vector vaccine, the immunity does not come from replication of a complete virus, triggering all the arms of the immune system (‘complete’ immunity), but essentially from an antibody response to the VP2 antigen of the IBD virus expressed by the recombinant HVT vector. That way, when considering protection against the Gumboro virus, the useful part of the immune response is mostly of the humoral type. Protection against Gumboro Disease comes from the expression of the VP2 by rHVT-VP2 -infected cells, and is a much slower process. Onset of immunity against IBD requires much more time than for MD.
Finally, it is established that the level of protection induced by rHVT-VP2 vaccines is dependent on the IBDV strain challenging the chickens. As has already been said, protection comes from stimulation of the immune system with a specific VP2 protein and not with the whole virus, making this protection more effective against field viruses carrying a similar VP2.
As protection is partial, the IBDV strains that are different will escape vaccine protection and this will favour the emergence of new variant IBDV strains. These new strains will have the ability to break through MDA earlier than before, meaning that the challenge will come earlier, which will then increase virus pressure and allow Gumboro Disease to become out of control.
Immune-complex IBD vaccines
Immune-complex IBD vaccines are prepared from live attenuated IBDV strains of the intermediate plus type, mixed in with specific anti IBDV serum to regulate the safety and release of the vaccine once the MDA levels of the bird are reduced. A correct balance between the IBD virus and the anti IBDV antibodies is of crucial importance for the efficacy and safety of these vaccines.
These vaccines have the ability to fully colonise the bursa and to protect against all field IBD viruses. The ‘take’ depends on the quality of application - the objective of a proper vaccine administration is not only to reach every chicken, but also to ensure that the full dose is received by each of them.
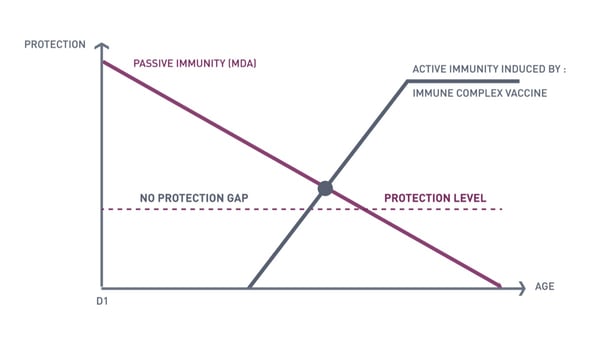
The capacity of a vaccine strain to overcome MDA is one of the key advantages of the immune-complex vaccines. From the first day, the MDA levels will decrease. This decrease is variable according to the starting MDA level at hatch. The vaccine take occurs when the MDA level decreases to a point that allows the vaccine virus to be released and to reach the bursa of Fabricius. From this moment, the vaccine strain will replicate in the bursa of Fabricius, and the chicken will be immunized against any type of IBD virus (Active Immunity).